 Since we are now fully comfortable with “smart” everything, it should be no surprise that 3D printing has taken center stage in the tech world. It’s easy to imagine 3D-printed machine parts, prototype models, or even toys, but it might be harder to accept 3D-printed foods, implantable medical devices, cosmetics, drugs, and even human tissue. Too futuristic? Not really. The technology to 3D-print, U.S. Food and Drug Administration (“FDA”)-regulated products is, in large part, already here and rapidly progressing.
Since we are now fully comfortable with “smart” everything, it should be no surprise that 3D printing has taken center stage in the tech world. It’s easy to imagine 3D-printed machine parts, prototype models, or even toys, but it might be harder to accept 3D-printed foods, implantable medical devices, cosmetics, drugs, and even human tissue. Too futuristic? Not really. The technology to 3D-print, U.S. Food and Drug Administration (“FDA”)-regulated products is, in large part, already here and rapidly progressing.
Yet, as technology continues to develop, questions arise as to whether, and how, the FDA regulatory framework will keep pace to impose the same safety, quality, and efficacy standards to 3D-printed foods, drugs, cosmetics, and medical devices that currently apply to traditionally manufactured goods. How FDA chooses to deal with 3D-printed products will significantly impact not only barriers to market-entry, but also post-marketing enforcement risks. Similarly, even assuming an FDA-regulated 3D-printed product is successfully brought to market in accordance with FDA standards, manufacturers must still assess options and potential challenges associated with protecting their intellectual property.Continue Reading 3D Printing Series: Be Right Down — Printing My Makeup
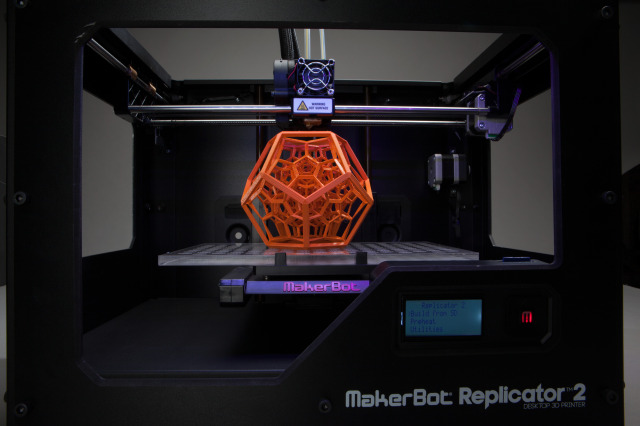 We agree – the Jetsons era has indeed arrived. Beyond the days of “smart” everything, now 3D printing has taken center stage in the tech world. While it is not so farfetched to imagine
We agree – the Jetsons era has indeed arrived. Beyond the days of “smart” everything, now 3D printing has taken center stage in the tech world. While it is not so farfetched to imagine 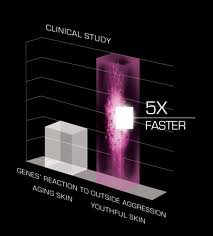 Who doesn’t want young-acting skin? We’re not talking about the way skin acted in the zits-on-picture-day years, but rather the dewy glow of innocence – the Code of Youth.
Who doesn’t want young-acting skin? We’re not talking about the way skin acted in the zits-on-picture-day years, but rather the dewy glow of innocence – the Code of Youth.